Chemical Effects of Electric Current Class 8 Science Chapter 14: Chemical Effects of Electric Current Class 8
Chemical Effects of Electric Current Class 8 Science Chapter 14 Chemical Effects of Electric Current class 8 notes, MCQs, questions answers, concept map and revision material for CBSE students.
Table of Contents
📝 Chemical Effects of Electric Current Class 8 Science Chapter 14 CHAPTER NOTES
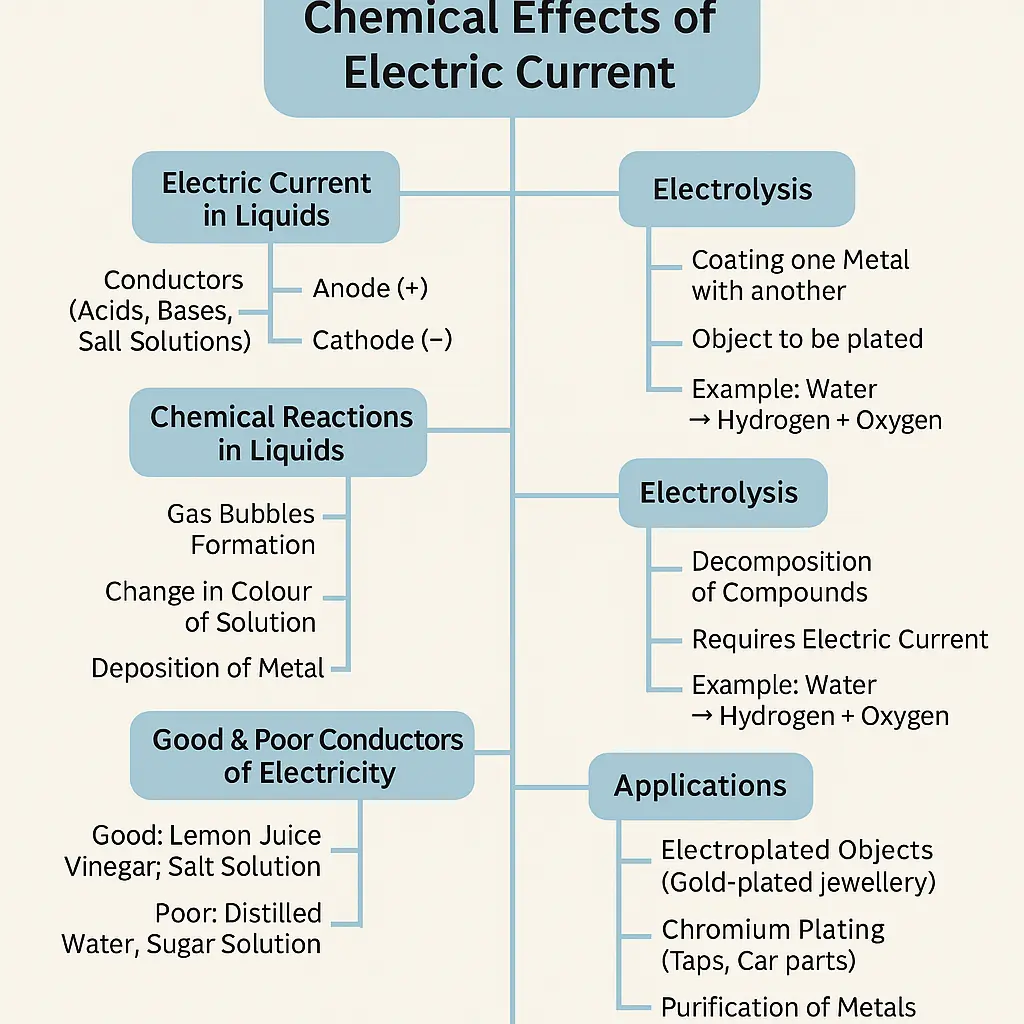
🔹 1. Electric Current in Liquids
- Electric current can flow through liquids.
- Liquids that allow electricity to pass are called conducting liquids.
- Most conducting liquids are solutions of acids, bases, or salts.
Examples of conducting liquids:
- Lemon juice
- Vinegar
- Salt solution
Poor conductors:
- Distilled water
- Sugar solution
- Oil
🔹 2. Conductors and Insulators
- Conductors: Allow electric current to pass.
- Insulators: Do not allow electric current to pass.
| Conductors | Insulators |
|---|---|
| Salt solution | Distilled water |
| Lemon juice | Sugar solution |
| Vinegar | Oil |
🔹 3. Chemical Effects of Electric Current
When electric current passes through a conducting liquid, it can produce chemical changes.
Chemical effects include:
- Formation of gas bubbles
- Change in colour of the solution
- Deposition of metal on electrodes
These changes prove that electric current causes chemical reactions in liquids.
🔹 4. Electrodes
- Electrodes are metal rods or plates dipped in a solution.
- They help in passing electric current through liquids.
Types of electrodes:
- Anode (+) → Connected to positive terminal
- Cathode (–) → Connected to negative terminal
🔹 5. Electrolysis
- Electrolysis is the process of breaking down a compound using electric current.
- It causes chemical decomposition.
Example:
- Water breaks into:
- Hydrogen gas
- Oxygen gas
🔹 6. Electroplating
- Electroplating is the process of coating one metal with another using electric current.
Example:
- Gold-plated jewellery
- Chromium-plated taps
Advantages of electroplating:
- Prevents rusting
- Improves appearance
- Saves cost
🔹 7. Applications of Chemical Effects of Electric Current
- Electroplating of metals
- Purification of metals
- Manufacturing of chemicals
- Decorative coating of objects
❓ Chemical Effects of Electric Current Class 8 Science Chapter 14 QUESTION–ANSWERS
🔹 Very Short Answer Questions (1 Mark)
Q1. What is a conducting liquid?
Ans: A liquid that allows electric current to pass through it is called a conducting liquid.
Q2. Name one poor conductor of electricity.
Ans: Distilled water.
Q3. What is electroplating?
Ans: Electroplating is coating one metal with another using electric current.
Q4. Which electrode is connected to the negative terminal?
Ans: Cathode.
🔹 Short Answer Questions (2–3 Marks)
Q1. What are the chemical effects of electric current?
Ans:
The chemical effects of electric current are:
- Formation of gas bubbles
- Change in colour of solution
- Deposition of metal
Q2. Why is distilled water a poor conductor of electricity?
Ans:
Distilled water does not contain dissolved salts or ions, so it cannot conduct electricity easily.
Q3. What is electrolysis? Give one example.
Ans:
Electrolysis is the process of breaking a compound using electric current.
Example: Water breaks into hydrogen and oxygen gases.
🔹 Long Answer Questions (5 Marks)
Q1. Explain the process of electroplating.
Ans:
Electroplating is the process of coating one metal over another using electric current. The object to be plated is connected to the negative terminal (cathode), and the metal to be coated is connected to the positive terminal (anode). When electric current passes through the solution, metal particles get deposited on the object. Electroplating is used to prevent rusting, improve appearance, and reduce cost.
Q2. Describe an experiment to show the chemical effect of electric current.
Ans:
Take a circuit with a battery, switch, bulb, and two electrodes. Dip the electrodes in a salt solution. Switch on the circuit. The bulb glows and gas bubbles appear near the electrodes. This shows that electric current produces chemical changes in the solution.
✅ Chemical Effects of Electric Current Class 8 Science Chapter 14 MCQs – Chemical Effects of Electric Current (Class 8)
Choose the correct option
1. Which type of liquids generally conduct electricity?
A. Pure water
B. Salt solutions
C. Oil
D. Sugar solution
✅ Answer: B
2. Distilled water is a poor conductor because it:
A. Contains acids
B. Has no dissolved salts
C. Is too cold
D. Is colourless
✅ Answer: B
3. Which of the following is a conducting liquid?
A. Oil
B. Sugar solution
C. Lemon juice
D. Distilled water
✅ Answer: C
4. When electric current passes through a solution, which effect is observed?
A. Magnetic effect
B. Heating effect
C. Chemical effect
D. Mechanical effect
✅ Answer: C
5. Which gas is released during electrolysis of water?
A. Nitrogen
B. Carbon dioxide
C. Hydrogen and Oxygen
D. Oxygen only
✅ Answer: C
6. The electrode connected to the negative terminal is called:
A. Anode
B. Cathode
C. Fuse
D. Switch
✅ Answer: B
7. Which electrode is connected to the positive terminal?
A. Cathode
B. Neutral
C. Anode
D. Earth
✅ Answer: C
8. Electrodes are usually made of:
A. Wood
B. Plastic
C. Metals
D. Rubber
✅ Answer: C
9. Which of the following is a chemical effect of electric current?
A. Heating of wire
B. Rotation of fan
C. Change in colour of solution
D. Lighting of bulb
✅ Answer: C
10. Electrolysis is the process of:
A. Mixing chemicals
B. Heating liquids
C. Chemical decomposition using electricity
D. Purification by filtration
✅ Answer: C
11. What happens near electrodes during chemical reactions?
A. Ice formation
B. Gas bubbles
C. Sound
D. Light
✅ Answer: B
12. Electroplating is mainly used to:
A. Increase weight
B. Improve appearance and prevent rusting
C. Reduce conductivity
D. Melt metals
✅ Answer: B
13. Which metal is commonly used for plating taps?
A. Copper
B. Iron
C. Chromium
D. Aluminium
✅ Answer: C
14. In electroplating, the object to be plated is connected to:
A. Positive terminal
B. Negative terminal
C. Switch
D. Battery case
✅ Answer: B
15. Which solution is used for electroplating?
A. Sugar solution
B. Oil
C. Salt of the plating metal
D. Distilled water
✅ Answer: C
16. Which of the following is a poor conductor?
A. Vinegar
B. Salt solution
C. Distilled water
D. Lemon juice
✅ Answer: C
17. Which effect of electric current is used in electroplating?
A. Heating
B. Magnetic
C. Chemical
D. Mechanical
✅ Answer: C
18. Which of the following is NOT a chemical effect?
A. Gas bubbles
B. Metal deposition
C. Colour change
D. Heating of wire
✅ Answer: D
19. Which material is electroplated to reduce rusting?
A. Plastic
B. Wood
C. Iron
D. Rubber
✅ Answer: C
20. Why is electroplating preferred over using pure gold?
A. Gold is weak
B. Gold is cheap
C. It reduces cost
D. It increases weight
✅ Answer: C
21. Which substance increases conductivity of water?
A. Sugar
B. Salt
C. Oil
D. Sand
✅ Answer: B
22. What is required for electrolysis?
A. Heat
B. Magnet
C. Electric current
D. Pressure
✅ Answer: C
23. Which of the following is an application of chemical effects of electric current?
A. Electric fan
B. Electric heater
C. Electroplating
D. Electric bell
✅ Answer: C
24. What change is observed near electrodes during electrolysis?
A. Sound
B. Light
C. Gas bubbles
D. Heat
✅ Answer: C
25. Which of these liquids conducts electricity the least?
A. Vinegar
B. Lemon juice
C. Salt solution
D. Distilled water
✅ Answer: D
26. What is the main purpose of electroplating iron objects?
A. Decoration only
B. Prevent corrosion
C. Increase weight
D. Reduce size
✅ Answer: B
27. Which term refers to the flow of electric current through liquids?
A. Magnetism
B. Electrolysis
C. Conduction
D. Radiation
✅ Answer: B
28. Which ion movement causes current in liquids?
A. Electron flow
B. Proton flow
C. Ion movement
D. Neutron flow
✅ Answer: C
29. What kind of effect produces metal deposition?
A. Magnetic
B. Heating
C. Chemical
D. Mechanical
✅ Answer: C
30. Which chapter studies electroplating?
A. Force and Pressure
B. Friction
C. Chemical Effects of Electric Current
D. Light
✅ Answer: C
